According to a new report from the Center of Technology Innovation at the Brookings Institution, private sector investment in overall health research and development (R&D) exceeds $150 billion annually, but only $5.9 billion of this is focused on diseases that primarily affect low- and middle-income countries (LMICs). Some of these diseases—such as HIV/AIDS, malaria, tuberculosis, diarrheal diseases, and pneumonia and meningitis—impose very large disease burdens as measured in Disability-Adjusted Life Years (DALYs) lost to the disease, and this burden is disproportionately borne by the populations of LMICs.
Figure 1. Public, private, and philanthropic neglected disease R&D spending and DALYs, 2015
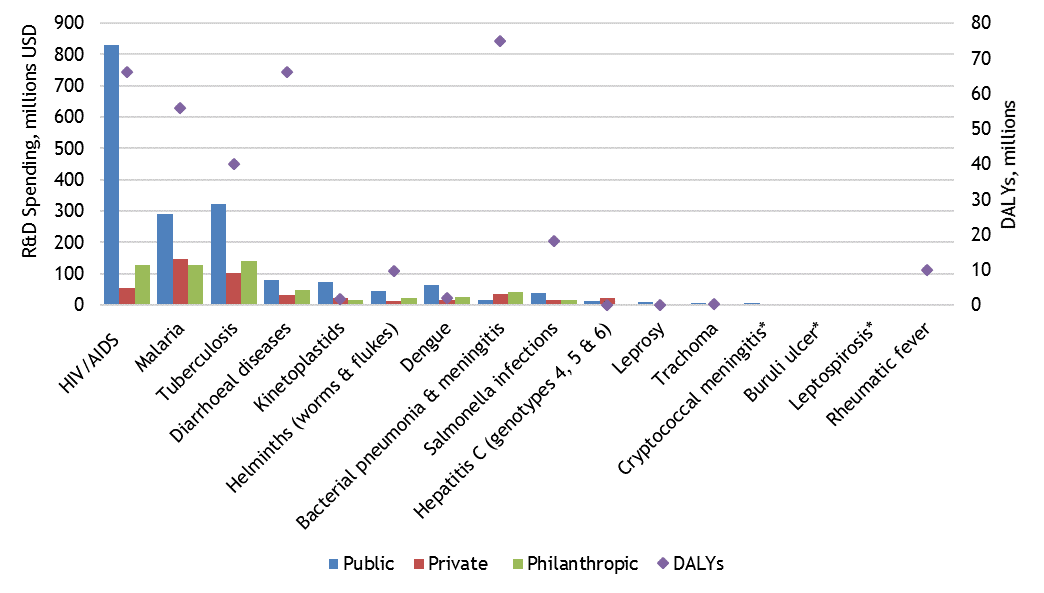
Source: Chapman et al., 2016
* Information on 2015 DALYs not available
Health R&D requires long-term investment, with financial returns from research and clinical trials several years or even decades in the future. The returns are also conditional on scientific success, regulatory approval, and favorable market conditions. Global health R&D investment aimed at high-burden diseases which primarily affect LMICs may also be subject to greater uncertainty than investments targeting diseases more prevalent in high-income markets.
Since private sector R&D investment choices are expected to reflect the most profitable use of funds or the most comfortable risk-return tradeoffs, it is unsurprising that global health R&D attracts a relatively small share of private R&D funding, and that most funding for global health R&D thus arises from the public and philanthropic sectors (Figure 1). There are, however, examples of privately funded R&D, blended financing, and public-private partnerships targeting diseases in LMICs. This suggests possibilities at the margin for catalyzing more private sector investment by increasing returns, lowering risk, or overcoming institutional disincentives for private R&D funding. A recent EPAR review examines the evidence in the literature for five hypothesized disincentives to private sector investment in global health R&D, and looks at examples of policy incentives that may help promote private sector investment.
What factors affect private global health R&D investment?
Our review aimed to analyze evidence from the literature on particular cost, revenue, and market issues affecting private sector investment in global health R&D. We identified five key factors hypothesized to affect private sector investment: scientific uncertainty; uncertain, unstable, or weak policy environments; limited revenues and market uncertainty, high fixed and sunk costs, and downstream rents from imperfect markets. In a review of 285 sources published in the past 15 years that discuss private investment in 47 individual diseases, we analyzed and recorded information on which factors are most commonly discussed as disincentives to private sector global health R&D investment.
Scientific uncertainty alone is infrequently mentioned as a factor limiting private global health R&D investment, though the time and likelihood of success affects costs. Some sources report that scientific complexity is a major factor affecting R&D investment choices, but this concern is not limited to global health R&D. Only four studies reviewed emphasize complexity of research, access to existing research and the limited volume of existing knowledge as specific factors influencing private R&D investment decisions. One study, which examines rare diseases and orphan drug development, finds that diseases with more existing and available research, and therefore less scientific uncertainty, are more likely to spur drug development. Another study reports that the greater complexity of major global health diseases and markets makes developing vaccines more challenging and requires additional investments in time and money.
Uncertain, unstable, or weak policy environments exist in countries with poor health delivery systems, poor health governance, uncertain regulatory environments, weaker macroeconomic conditions, or higher geopolitical risk. Industry reports frequently mention risks related to policy environments as deterrents to private investment in global health R&D. Most sources offer little specificity. Uncertainty related to regulatory environments and intellectual property rights is more commonly cited as a concern than general macroeconomic volatility. One study mentions that uncertain regulatory environments in LMICs may delay product approval. Other sources describe a lack of regulatory cooperation, a lack of involvement of regulatory agencies in public-private partnerships (PPPs), and high regulatory costs that may limit the impact of small innovative R&D firms as barriers to investment.
Low or uncertain prices for global health products in LMICs relative to prices for products targeting the U.S. or other HICs, limiting revenues and creating market uncertainty, can deter private global health R&D investment. Consumers and providers in LMICs, despite a high burden of disease, have a relatively low ability to pay. Annual spending on drugs is $240 per person in OECD countries compared to LMICs $20 per person. The problem of predicting revenues is further complicated because the end-user of medical products is not always the buyer. In many high-income countries, the intermediary purchaser of drugs, vaccines, or diagnostics is a private insurance company or a government institution. In many LMICs, development agencies and philanthropic organizations, such as Gavi or the Bill & Melinda Gates Foundation, act as intermediaries, contributing more than $35 billion to health programs in LMICs in 2014.
Though expected revenues from global health R&D may be low or uncertain, costs are often high and incurred upfront with certainty. The high costs of drug development, including many costs that cannot be repurposed (sunk costs), are cited by many sources as limiting all health R&D, with several sources noting the large disease-specific cost of drug or vaccine development. Studies report that the costs of bringing a drug to market range from $802 million to $2.2 billion, though there is some criticism that these numbers are inflated. One source suggests that, among other factors, the growing size of clinical trials and increasing clinical development costs may have contributed to the declining number of fundamentally new products since the mid-1990s.
Downstream rents from imperfect markets occur when it is less costly to “buy rather than make”, and firms with downstream purchasing power can purchase patent and intellectual property rights at a lower cost than investing in their own health R&D if they have more market power than upstream developers. Philanthropic and public funding may also subsidize upstream research in ways that disincentivize new private R&D funding. One source reports that it is common industry practice for a pharmaceutical company to either purchase another company or company’s products in order to secure a patent. Another source notes that although patents can be an effective tool in incentivizing private investment in health R&D, the current patent structure may actually encourage firms to spend more money on marketing than R&D.
 Policy and market evolution
Policy and market evolution
Of the five factors we reviewed, our literature review found the most discussion of limited revenues /market uncertainty as a disincentive to private sector investment in global health R&D. High costs were also commonly mentioned, but are more of a general characteristic of health R&D and less specific to investment in R&D focused on LMICs.
We find evidence that the current health R&D market structure is characterized – and likely constrained - by specialization, high entry costs, regulatory rents and privately held information; a result of both the nature of disease research and the policy environment. In a perfectively competitive market, in a situation where the vast majority of private investment is flowing into high income country (HIC) health R&D, at some point the marginal return to a dollar invested in LMIC health R&D would exceed the marginal returns to further HIC health R&D investment (so long as LMIC health R&D was at all profitable). But in an imperfectly competitive market this threshold may not be realized.
Looking forward, we expect the attractiveness of licensing upstream research rather than conducting R&D internally may increase as more computing and data analysis occur in biotech companies relative to the physical science labs of traditional pharmaceutical companies. Customer and market data collected remotely, via social media, through internet searches, or through other means (utility payments, bank transactions, etc.) contain information that has commercial value by informing market opportunities. And as the industry evolves further from a “chemical compound configuration” to a “biotech/biopharmaceutical configuration” resting on “sophisticated informatics and big data infrastructure,” (R&D Magazine, 2016), the potential to easily share market, customer, and health knowledge expands, but so does the opportunity to monopolize it, depending on the policies and other incentives facing private investors.
Though a variety of policy tools exist to promote private sector investment in R&D, including push mechanisms (public research funding, R&D tax credits) and pull mechanisms (advance purchase commitments, orphan drug programs, priority review vouchers, and wild-card patent extensions), evidence of effectiveness is mixed. Our full report goes into greater detail on the evidence of these mechanisms in increasing private sector global health R&D spending.
Finally, to the extent that health science and market data are relatively limited for global health R&D, there is reason to speculate that as the pharmaceutical industry evolves an even smaller share of investment will be directed at diseases prevalent in LMICs. Both industry experts and the literature lament the limited market data available to better assess potential market outcomes – yet despite potential industry-wide gains, there is no clear incentive for any individual firm within this sector to either fund or contribute to such a data service. While past policy debates in the area of privately funded global health R&D have long centered on the role of patents (that are often time limited, have distorting incentives, and are unevenly enforced in LMICs), future alternative approaches for rewarding scientific innovation might be to undertake efforts to compile market information and make market data fully public.
By Pierre Biscaye, David Coomes, and Vivian Gor
Summarizing original EPAR research by Leigh Anderson, Carol Levin, Travis Reynolds, Pierre Biscaye, Karen Chen, David Coomes, Elan Ebeling, Vivian Gor, Trygve Madsen, and Emily Morton